|
|
|
|
|
|
|
|
|
the
stratosphere over the southern hemisphere was unusually disturbed this year
by the wind, causing the hole to split into two separate holes. |
WHILE OZONE at
ground level is considered a pollutant, the layer of ozone high in the
stratosphere is vital to life because it blocks dangerous radiation coming
from the sun. Thinning of the ozone layer could lead to a rise in skin
cancer, experts warn. Aerosols and other chemicals are blamed for the
thinning, and treaties banning those ingredients are expected to help the
layer recover over time. This year’s improvement was attributed to warmer than normal temperatures around the edge of the polar vortex, or circular wind pattern that forms annually in the stratosphere over Antarctica, according to Paul Newman, a lead ozone researcher at NASA’s Goddard Space Flight Center in Greenbelt, Maryland. Craig Long, a meteorologist at the NOAA Climate Prediction Center, said the stratosphere over the southern hemisphere was unusually disturbed this year by the wind, causing the hole to split into two separate holes. |
|||
|
|
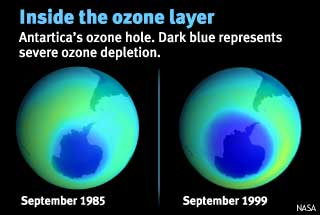
In 2001 the
Antarctic ozone hole reached a maximum size of more than 10.2 million square
miles, larger than the entire area of North America, including the United
States, Canada and Mexico combined. In the year 2000, it briefly approached
11.5 million square miles. The last time the ozone hole was as small as it
is this year was in 1988, and that was also due to warm temperatures. Newman explained that while “chlorine and bromine chemicals cause the ozone hole, the temperature is also a key factor in ozone loss.” The coldest temperatures over the South Pole occur in August and September. Thin clouds form in these cold conditions, and chemical reactions on the cloud particles help chlorine and bromine gases to rapidly destroy ozone. By early October, temperatures typically start to warm and the ozone layer starts to recover. |
|||
|
|
|
|
![]()
 |
||
|
|
What is the ozone? Earth's defense against solar radiation The ozone is an atmospheric gas which filters out a harmful form of solar radiation known as ultraviolet radiation. About 90% of the ozone in our atmosphere is contained in the stratosphere (the region from about 30,000 feet to 180,000 feet above the Earth's surface). |
|
|
|
How is it made? Ozone is produced by intense ultraviolet (UV) radiation in the upper stratosphere, which causes oxygen molecules (O2) to reform as ozone molecules (O3). The ozone molecule spends most of its life absorbing UV. |
|
|
|
How is it destroyed? Ozone is destroyed when it reacts with one of a variety of chemicals in the stratosphere such as chlorine, nitrogen, bromine or hydrogen. Such chemicals come primarily from man-made chlorine and bromine compounds produced for refrigerants, aerosol sprays, and solvents. |
|
|
|
Why study at the poles? A number of factors found only at the poles increase the likelihood of ozone depletion. As the air in the stratosphere cools and descends during the winter, strong winds create a vortex around each pole. This air is effectively isolated the rest of the atmosphere. Within this vortex form polar stratospheric clouds (PSCs). Studies indicate that the chemical composition of these stratospheric clouds - and the extremely low temperature - provide the perfect environment for breaking ozone molecules. |
|
|
|
Antarctica's ozone hole Ozone levels over Antarctica fall to abnormally low values between August and late November, destroying about 95% of the ozone in the lower stratosphere. In 1987, and from 1989-95, the hole covered the entire continent and part of the surrounding ocean. There is currently no ozone hole at the North Pole, but a recent study determined that ozone losses of over 60 percent occurred in the Arctic stratosphere at an elevation near 60,000 feet during the winter of 1998/1999. |
|