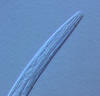
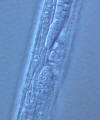
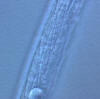
Family Rhabditidae
Rev 05/28/2025
Classification:
- Chromadorea
Rhabditida
Rhabditina
Rhabditoidea
Rhabditidae Oerly,
1880
Rhabditid nematodes are very abundant in all types of soil and sediments of
freshwater. They play important ecological roles mainly as primary
consumers—their freeliving forms display saprophagous or bacteriophagous feeding
habits—but also as animal parasites, in particular enthomopathogenic forms.
From a systematic point of view, rhabditids are a difficult nematode group
whose classification has been muche discussed and whose diversity is far from
being well known (Abolafia and Pena-Santiago, 2007). They are mainly
distinguished by their lip characteristics and shape of appendages, male tail
characters, especially the number and arrangement of genital papillae,
presence/absence and shape of bursa, shape of spicule and gubernaculum, and
female/hermaphrodite tail morphology (Sudhaus, 2011; Scholze & Sudhaus, 2011).
Phylogenetically, the family is not monophyletic; other families and
non-Rhabditidae clades, e.g., strongyloidids, are located between two
independent ‘Rhabditidae’ groups of genera groups (e.g., van Megen et al., 2009;
Smythe et al., 2019).

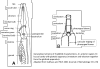
| Generalized Characteristics of anterior and
posterior regions of Rhabditidae (from Sudhaus and Fitch, 2001) |
 |
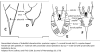 |
Basic Body Plan of Nematodes in the Family Rhabditidae ( from
Sudhaus, 2014)
(1) Adults about 1 mm long; females slightly longer than males on average.
(2) Six lips with sensilla in two concentric circles around the mouth opening;
small amphid pores at the base of the lateral lips; lateral deirids encircling
the isthmus posterior to the nerve ring; lateral postdeirids located in the
posterior half of the body.
(3) Stoma tube-like with prismatic cross section; a short cheilostom in the lip
region, a gymnostom of half the stoma length, a stegostom enveloped by a sleeve
of pharyngeal tissue forming; stoma terminating in a glottoid apparatus bearing
cuticular structures like denticles.
(4) Pharynx tripartite, muscular with a corpus (with anterior radial tubes for
reflux of water during the filtering of bacteria), a narrower cylindrical
isthmus and a terminal bulb with three rhabditoid valves (the grinder) to crush
bacteria, followed by two cuticularized chambers (double haustrulum) and a
funnel-like cardia opening to the intestine.
(5) Secretory-excretory system H-shaped with an osmoregulation function
and, at least in the dauerlarva, shows pulsations of a tiny ampulla at the
beginning of the duct.
(6) Females with a midbody vulva with a circular opening and two opposed
(amphidelphic) genital branches, which are homodromously (within the growth zone
of the ovaries) reflexed to the dorsal, the anterior branch right of intestine,
the posterior branch on the left side.
(7) Tails of juveniles, and probably also tail of the female, conical tapering.
(8) Males monorchic; testis to the right of intestine and ventrally reflexed.
(9) Posterior end of the male with a series of nine bilateral pairs of sensitive
genital papillae subventrally, presumably three of them anterior of the cloaca,
and two postcloacal papillae (in positions five and seven) oriiented more
sublaterally/dorsally. The similar arrangement of papillae (including the
position of the first papilla far anterior of the cloaca) is considered
symplesiomorphic.
(10) A pair of phasmids posterior of genital papillae with a ventral opening and
circumcloacal sensilla consisting of one on the precloacal lip and a pair
postcloacally.
(11) Copulation involves the male to curl its posterior end around the female
body; often a small gelatinous copulatory plug is deposited to seal the vulva.
Spicules separate and gubernaculum unforked.
(12) Life cycle includes an ecologically important dauerlarva, a developmental
alternative to the normal third-stage juvenile, which, by retaining the cuticle
of the preceding juvenile stage and living from intestinal reserves, can
withstand periods of unfavorable conditions.
(13) Gonochoristic reproduction, presumably longliving and oviparous, steadily
producing eggs over a longer period..
Under some conditions eggs are retained in the body of older females.
The female dies, the eggs hatch, and juveniles feed on bacteria that are
decomposing the maternal cadaver. The phenomenon has been termed "bagging"
and "endotokia matricida". It has been variously interpreted as the result
of diminished strength of the vaginal muscles that would be involved in allowing
passage of the egg, and the resulting death of the worm (hence matricida), and
also as a facultative vivipary, survival adaptation that provides resources for
the juveniles (Chen and Caswell-Chen, 2004; Seurat, 1914).
 |
Bagging or Endotokia Matricida: Juveniles
beginning to emerge from the depleted maternal cadaver of a rhabditid
nematode
Photograph by Jonathan Nivens |
| |
Copulation in the Rhabditidae:
Various copulatory positions can be distinguished in
nematodes. The most common form is the “spiral form” or “radial form” in
which the male winds the posterior portion of its body spirally around,
and perpendicular to, the body axis of the female. The stability of the male in
this form of copulation by ventromedian supplements that are precloacal. With
the evolution of the bursa, as, for example, in the Rhabditidae, the “parallel
form” of copulatory position became possible; males could be stabilized on the
surface of the female’s body with the aid of a “suction-cup-like” bursa and
supported by the secretion of cloacal glands.
The position of the female vulva does not appear to
influence the form of copulation; howvere, it would seem that the spiral form of
copulation would be difficult if the vulva is very posterior in position. The
evolution of the bursa and the arrangement of the bursal papillae must be of
considreble importance in copulatory behavior. Although the primary function of
the bursal papillae is sensory, they also allow the formation and support of a
broad bursal velum whic facilitates grasping and orientation on the female body
in the parallel form of copulation.
The shape and size of spicules must also be adapted to
the copulatory position. In the spiral form with the absence of a bursa,
curved spicules should provide an advantage in penetration of the vulva.
On the other hand, in parallel copulation as in most Rhabditidae, straighter and
longer spiculse are probably an advantage.
In rhabditid species with a closed bursa, the suction
effect between the busra and female body is optimized by secretion of copulatory
cement from the caudal glands. When copulation isds completed, the cement
remains covering the vulva as a copulatory plug which may remain in place until
egg production commences (Sudhaus and Fitch, 2001)
Most soil Rhabditidae are considered to be
bacterial-feeding r-strategists that respond rapidly to environmental enrichment
and increase in bacterial biomass.
Bacteria are ingested in the soil solution; the pharyngeal corpus has
anterior radial tubes for reflux of water during the filtering of bacteria and a
terminal bulb with three rhabditoid valves (the grinder) to crush ingested
bacteria (Sudhaus, 2014).
Other species are parasites of invertebrates or have commensal relationships
with insects (Morand et
al., 2004).
Several different genera are associated with molluscs, including
Rhabditis,
Caenorhabditis
and Phasmarhabditis.
Unlike true mollusc parasites
(e.g. Agfidae and Angiostomatidae), Phasmarhabditis spp.
are facultative parasites that live on compost, leaf litter, slug faeces, and
dead earthworms and insects. Some have
a necromenic strategy in relation to the larger slug species.
Caenorhabditis elegans can
invade the intestine of molluscs and exit with feces.
Phasmarhabditis
hermaphrodita has
been developed as a commercial biological control agent of slugs and snails
(Pieterse et al., 2017).
References
Abolafia, J and Pena-Santiago, R. 2007. Nematodes of the Order Rhabditida from
Andalucía Oriental, Spain. The Genera Protorhabditis (Osche, 1952) Dougherty,
1953 and Diploscapter Cobb, 1913, with Description of P. spiculocrestata sp. n.
and a Species Protorhabditis Key.
Journal of Nematology, 39:263-274.
Chen, J, Caswell-Chen, E.P. 2004. Facultative vivipary is a life-history
trait in Caenorhabditis elegans. J. Nematology 36:107-113.
Morand, S., Wilson, M.J. & Glen, D.M. (2004) Nematodes
(Nematoda) parasitic in terrestrial gastropods.
pp. 525–557 in Barker, G.M. (Ed.) Natural
enemies of terrestrial molluscs. Wallingford, CABI
Publishing.
Pieterse, A., Malan, A.P., Ross, J.L. 2017.
Nematodes that associate with terrestrial molluscs as definitive hosts,
including Phasmarhabditis
hermaphrodita (Rhabditida: Rhabditidae) and its
development as a biological molluscicide. J. Helminthol. 91:517-527.
Scholze, V.S. & Sudhaus, W. 2011.. A pictorial key to current genus groups of
“Rhabditidae”. Journal of Nematode Morphology and Systematics 14: 105-112.
Seurat, L.G. 1914. Sur un cas d’endotokie matricide chez un oxyure.
Comptes-Rendues de la Société de Biologie 76:850-853.
Smythe, A.B., Holovachov, O. & Kocot, K.M. 2019.. Improved phylogenomic
sampling of free-living nematodes enhances resolution of higher-level nematode
phylogeny. BMC Evolutionary Biology 19, 121: DOI: 10.1186/s12862-019-1444-x
Sudhaus, W. (2011). Phylogenetic systematisation and catalogue of
paraphyletic “Rhabditidae” (Secernentea, Nematoda). Journal of Nematode
Morphology and Systematics 14, 113-178.
Sudhaus, W. 2014. 7.17 Order Rhabditina: "Rhabditidae". In: A.
Schmidt-Rhaesa (ed.): Handbook of Zoology. Gastrotricha, Cycloneuralia and
Gnathifera, Vol. 2: Nematoda. W. deGruyter, Berlin, Boston: 537–555.
Sudhaus, W. and Fitch, D. 2001. Comparative studies on the phylogeny and
systematics of the Rhabditidae (Nematoda). J. Nematology 33:1-70.
Sulston, J.E. and H.R. Horvitz. 1977. Post-embryonic cell lineages of the
nematode Caenorhabditis elegans. Developmental Biology
56:78:577-597.
Waterston, R.H. 1988. Muscle. Pp 281-335 in W.B. Wood (ed). The
Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory.
Go to Dictionary of
Terminology
Go to
Mesorhabditidae Menu
Go to Rhabditidae Menu
Go to Nemaplex Main Menu