- Tylenchida
Tylenchina
Tylenchoidea
Heteroderidae
Punctoderinae
- Globodera rostochiensis Wollenweber
Synonyms:
Globodera arenaria (Chizhov, Udalova & Nasonova, 2008)
Subbotin, Mundo-Ocampo & Baldwin, 2020
Until Stone (1973a) described a second cyst-nematode species (G. pallida)
that attacked potatoes, G. rostochiensis included both species. G.
pallida is very similar to G. rostochiensis, but differs from it in
several important morphological characters. Also, G. pallida
appears to be more aggressive than G. rostochiensis and is more
responsive to host root diffusate. When both species are present, G. pallida reproduces to a greater extent and predominates
population levels (Devine and Jones, 2003). The problem is exacerbated when
potatoes resistant only to G. rostochiensis are grown. Available
cultivars have only partial resistance to G. pallida and do not prevent
its increase. Globodera pallida is progressively replacing G.
rostochiensis in Britain (Trudgill et al., 2003). The genus
Globodera was established for the round cyst nematodes by Behrens (1975).
-
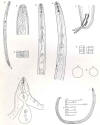
Females: Globodera rostochiensis females form cysts, which are
the bodies of dead female nematodes, which protect the eggs. Body globose,
spheroid, with a short
neck and no terminal cone.
New cysts are glossy-brown, rounded, and have a projecting neck (Stone,
1973b).
The stylet is straight to slightly curved and stylet knobs are rounded and
sloped posteriorly.
Ovaries are paired and large.
 Cuticle
thick, with superficial, lace-like pattern; D-layer present. Vulva terminal, of
medium length.
Cuticle
thick, with superficial, lace-like pattern; D-layer present. Vulva terminal, of
medium length.
Vulval area circumfenestrate; superficial tubercles near vulva.
No anal fenestration, but anus and vulva lying both in a "vulval
basin." Underbridge and bullae rarely present. All eggs retained in body
(no egg-mass).
Note: Globodera rostochiensis passes through a yellow stage before
rupturing root cortex. Globodera pallida remains
creamy white until dying and becoming a brown cyst. Mature adult
females on potato roots are golden-yellow in color.
 |
Mature cysts of Globodera rostochiensis
recovered from a potato field in Veracruz, Mexico
Photograph by Ignacio Cid del Prado Vera |
Males: Vermiform; body twisted into a C or S shape. Lateral
field with
four lines. Spicules greater than 30 µm in length, distally pointed. No
cloacal tubus. Tail short, hemispherical.
 Second-stage
juveniles: Stylet less than 30 µm long. Lateral
field with four lines. Esophageal
glands filling body cavity. Tail conical, pointed, with terminal half
hyaline. Phasmids punctiform.
Second-stage
juveniles: Stylet less than 30 µm long. Lateral
field with four lines. Esophageal
glands filling body cavity. Tail conical, pointed, with terminal half
hyaline. Phasmids punctiform.
 |
|
There are some morphological differences between G.
pallida and G. rostochiensis; e.g., in larval lip region, etc.
Several pathotypes of G. rostochiensis exist: British A, Dutch A,B,C.
For G. pallida: British B and E, Dutch D.
Pathotype classifications are based on ability of nematode to reproduce on
resistant cultivars.
|
Reported median body size for this species (Length mm; width micrometers; weight micrograms) - Click:
|
Globodera rostochiensis is widely distributed in potato-growing regions; genus probably originated in
Peru with Solanum tuberosum and other Solanum spp.
The potato cyst nematodes were originally discovered in Germany in 1913. By
that time, the potato cyst nematodes had spread throughout Europe (Wallace,
1964). During the 1960's and 1970's, Canada was found to have several areas of
potato cyst nematode infestation (Mai, 1977). Being 450 miles away, the
Vancouver Island location is the area closest to California where one of the
potato cyst nematodes is known to be established.
In the early 1970's, scientists in Mexico discovered an infestation of G.
rostochiensis in the state of Guanajuato (approximately 1,000 miles from the
California border), which is one of the major potato-producing regions in Mexico
(Alvarez, 1972).
Reported in South Africa in 1971 from an irrigated farm near Pretoria and
then from small farms around Johannesburg and Bon Accord. In Aprill 1999, it
was reported from the Ceres area in the western Cape Province of South
Africa (Knoetze et al., 2006).
In North America, G. rostochiensis was first discovered on Long
Island, New York (Nassau County) in 1941 after a potato grower noticed isolated
areas of poor plant growth (Mai and Lear, 1953). The nematode may have come in on equipment returned from Europe after WWI -
the field was an old
military camp which became a potato field. The discovery led to
establishment of a Federal
Quarantine based on the Golden Nematode Act.
Growers moved to Western New York following urbanization, and subsequent
outbreaks appeared; required 8-10 years to reach a detectable level.
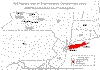
Golden Nematode Distribution in New York State, 1999
|
Based on information obtained at the International Potato
Center and literature review, the presence of Potato Cyst Nematode (PCN),
Globodera rostochiensis, has been reported in Austria, Australia,
Algeria, Belgium, Bolivia, Canada, Costa Rica, Chile, Czechoslovakia,
Denmark, Estonia, Finland, Germany, Greece, Holland, Hungary, India,
Iceland, Israel, Italy, Japan, Mexico, Morocco, New Zealand, Pakistan,
Panama, Peru, Philippines, Poland, Portugal, Sri Lanka, Spain, ex-Soviet
Union, South Africa, Sweden, United Kingdom, USA, Vancouver Island,
Venezuela and Yugoslavia.
In 16 of these countries there is also information about the races of
the nematode.
In 63% of the countries infested with PCN there are no data about
races.
The International Potato Center in Lima, Peru maintains a worldwide
collection of races and offers a free service for species and race
identification.
(Matos and Canto-Saenz, 1990) |
A-rated pest
in California Nematode Pest Rating System.
In general, those environments which favor potatoes and tomatoes also favor
the potato cyst nematodes. There are many ways in which the potato cyst
nematodes could be spread into potato- and tomato-producing regions in
California. Important among these is the spread of seed potatoes, plants, root
and soil material, either commercially or privately. Tools, implements,
automobiles and other equipment imported from overseas or from contaminated
areas in North America are all potential sources of infestation. A minute cyst
adhering to the underparts of a vehicle in mud would be very difficult to
detect. Although difficult to quantify, the probability of the potato cyst
nematodes being introduced into California is substantial. The probability of
them becoming established, once introduced, may be considerably less, but
nonetheless serious.
Feeding site establishment and development
typical of genus.
Nurse cell system is a multinucleate syncytium.
Very narrow range: potato, tomato, and some weeds.
Approximately 90 species of the genus Solanum are known to be
hosts.
Three commercial crops are hosts of potato cyst nematodes: potatoes,
tomatoes and eggplants (Mai and Lear, 1953).
In addition, numerous weeds are known to be hosts of these nematodes (Goodey
and Franklin, 1958; 1959). For example, bitter nightshade (S. dulcamara), silverleaf
nightshade (S. elaeagnifollum), hairy nightshade (S. sarracholdes), black
nightshade (S. nigrum), and jimsonweed (Datura stramonium), which
are all present in California, are hosts of the potato cyst
nematodes.
Several pathotypes of G. rostochiensis exist: British A, Dutch A,B,C.
Pathotype classifications are based on ability of nematode to reproduce on
resistant cultivars.
Within a host species, there are distinct cultivars that differ in their
susceptibility to various races of nematodes (Kort et al., 1977). This
differential susceptibility of cultivated varieties is the basis for one of the
primary means of controlling the potato cyst nematodes, that is, host plant
resistance.
Ecophysiological Parameters:
Survival, reproduction, and population dynamics of the potato cyst nematodes
can be greatly influenced by temperature, moisture, daylength, and edaphic
factors. In general, the potato cyst nematodes will survive in any environment
where potatoes can be grown. A period of 38-48 days (depending on soil
temperature) is required for a complete life cycle of the potato cyst nematodes
(Chitwood and Buhrer, 1945).
Eggs remain dormant within the dead female's body (the cyst) until the proper
stimulus to hatch is received (i.e. chemical stimuli released by host plant
roots). Potato cyst nematode eggs can remain dormant and viable within the cyst
for 30 years (Winslow and Willis, 1972). While in the dormant stage, the
nematodes are more resistant to nematicides (Spears, 1968).
When soil temperatures are warm enough (above 100C) (Ferris,
1957), and the proper hatching signals are received (Clark and Hennessy, 1984),
the second-stage juveniles hatch from the eggs, escape from the cyst and migrate
towards host plant roots. Egg hatch is stimulated by host root diffusate (60-80%) - only about 5% hatch
in water. Some eggs do not hatch until subsequent years.
Juveniles penetrate the roots and begin to feed within the root. Host plant
cells within the root cortex are stimulated to form specialized cells (syncytia)
which transfer nutrients to the nematodes. After feeding commences, the juvenile
grows and undergoes 3 more molts to become an adult.
Females grow and become round, breaking through the roots and exposing the
posterior portion of their body to the external environment.
Male juveniles remain active, feeding on the host plant until maturity, at
which time they stop feeding, become vermiform and seek females (Green, et al.,
1970).
Adult males do not feed. Sex is determined by food supply - more juveniles
develop into males under adverse conditions and heavy infestations.
Nematodes reproduce sexually; males are attracted to females by a pheromone
sex attractant. Nematodes may mate several times.
After mating, each female produces approximately 500 eggs (Stone, 1973b),
dies, and the cuticle of the dead female forms the cyst.
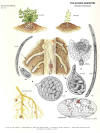 |
| Life cycle diagram by Charles S. Papp, CDFA |
Annual population decline in the absence of a host varies from 18% in cold
soils (Scotland) to 50% in warm soils, with an average decline rate about 30% -
so population decline follows this pattern: 100-70-50-35-23-etc.
Potato varieties resistant to G. rostochiensis have been
available since the 1970s and the resistance from Solanum tuberosum
spp. andigena CPC1673 (H1) has been a durable and effective way of
controlling G. rostochiensis in the UK. However, the extensive use
of varieties with this resistance has resulted in selection in favor of
G. pallida, which is now the most prevalent species (Varypatakis et
al., 2019).
|
Globodera rostochiensis effect on potato growth
(plant on right).
Photograph by Ulrich Zunke, University of Hamburg. |
 |
|

Roots of infected plants exhibit minute, white bodies, which are the
immature females that have erupted through the root epidermis. At
extremely high nematode densities, tubers may become infected, resulting
in the appearance of cysts on their surface.
|
In Scotland, 1 ton/acre is lost for every 20 eggs/g soil.
Supply of water and nutrients to upper plant is diminished.
The potato cyst nematodes are semi-endoparasites feeding on the roots
of plants.
No distinct host symptoms are associated with low populations, but as
populations increase symptoms appear. The plant will show poor growth in
small areas that enlarge with the continuous growing of potatoes. Plants
in these areas exhibit symptoms of water and/or mineral deficiency stress
with yellow leaves and wilting.
|
Exclusion and Quarantine
In countries that are free of the potato cyst nematodes, quarantines can help
prevent their introduction. In countries where their occurrence is localized,
quarantines can help prevent further spread. For example, quarantines imposed
after the initial discovery of G. rostochiensis on Long Island delayed
the spread of the nematode to the mainland (Evans and Stone, 1977). Once the
potato cyst nematodes have spread over a large area, quarantines are no longer
effective.
In the US, the Golden Nematode Act of 1948 created a quarantine on this
nematode. The Animal and Plant Health Inspection Service (APHIS) of the USDA
continues to enforce a quarantine on the interstate movement of restricted
articles from the New York State. Quarantine items include: soil, compost, peat,
manure, live plants, grass sod, plant crowns, roots for propagation, bulbs,
corms, rhizomes, root crops, small grain and soybeans (unless in approved
containers), hay and straw (unless in approved containers), plant litter, ear
corn (except shucked ear corn), used farm product containers, burlap bags, used
farm tools (unless free of soil), used cultivating or harvesting equipment
(unless free of soil), and seed potatoes. Potatoes for consumption grown in
fields certified free of potato cyst nematodes (or receiving applications of
required soil fumigants) may be transported if free of soil and moved in
approved containers. Those areas within New York which are known to be infested
with G. rostochiensis are regulated with respect to the intrastate
transport of restricted items.
As of 2014, 126,261 ha in 8 counties in New York State are under
regulation to curb the spread of G. rostochiensis and 2,422 have known
infestations and subject to control and mitigation programs (Whitworth et
al., 2018).
The discovery of G. rostochiensis in the US, and the consequent Golden Nematode Act,
had enormous impact on the development of Nematology. Many scientists
received their early training in Nematology through that program.
B.G.
Chitwood was one of the early directors of the Golden Nematode Lab.
In South Africa, strict quarantine measures were imposed to prevent the
spread of this nematode to other potato-producing areas. In both the South
African Plant Improvement Act (Act No. 53 of 1976) and Agricultural Pest Act
(Act No. 36 of 1983), G. rostochiensis is listed as a prohibited
pest. Distribution by means of seed potatoes is restricted by the
South African Seed Potato Certification Scheme of 15 May 1998, which
provides zero tolerance for infection.
tolerance for infection is
permissible (Knoetze et al., 2006).
Eradication
Eradication of the potato cyst nematodes once they are established in an area
seems unlikely. The extremely long survival period of cysts found in the soil
limits management options.
Management
Of all the crop pests worldwide, the potato cyst nematodes are among the most
difficult pests to control (Chitwood, 1951). Once established, they are
difficult to eradicate because the potato cyst nematodes have one of the highest
survival values for any organism, and can survive for over 30 years as eggs
protected by the durable cyst wall (Chitwood, 1951; Winslow and Willis, 1972).
Moreover, the build-up of nematode populations is slow, and their presence is
not easily detected; once the nematode populations increase to high levels,
drastic crop losses occur. One of the best methods of limiting spread once the
potato cyst nematodes are introduced into an area is to integrate control
measures. In particular, the use of pesticides, resistant varieties, and crop
rotation are necessary to prevent further spread in the United States (Evans and
Brodie, 1980).
Chemical Control - Chemicals used to control nematodes can be classified
according to their volatility as either fumigant nematicides or nonfumigant
nematicides. Depending on the concentration used, many fumigant nematicides are
general biocides that kill all living organisms in the soil, including
nematodes, fungi, bacteria, plants and insects. In contrast, many nonfumigant
nematicides are more specific and may not be
phytotoxic at field concentrations.
Resistant Varieties -
Resistance to G. rostochiensis pathotypes Ro1 and Ro2 is
conferred by the H1 gene. Current evidence suggeste that the H1 gene may
also confer resistance to G. ellingtonae because many cultivars
resistant to G. rostochiensis are also resistant to G.
ellingtonae (Whitworth et al., 2018). The H1 gene is derived from S.
tuberosum ssp. andigena and is located on chromosome 5 of potato.
It has been used extensively to provide a high level of durable resistance
against G. rostochiensis pathotypes Ro1 and Ro4 within potato
cultivars worldwide (Ernst et al., 2002).
Another nematode resistance gene, Gro1 from Solanum spegazzinii
confers resistance to G. rostochiensis pathotypes Ro1-5 and is
located on chromosome 7 of potato (Ernst et al., 2002).
Ernst and colleagues characterized the Hero gene of tomato, a
wide-spectrum nematode resistance gene that confers a high level (95%) of
resistance against all pathotypes of G. rostochiensis and > 80% resistance
to G. pallida.. The Hero gene was introgressed into tomato
cultivar LA1792 from the wild species, Solanum pimpinellifolium
LA121 by Ellis and Maxon-Smith (1971). In a survey of Japanese tomato
cultivars using PCR-RFLP, the Hero-A gene was detected in 22
cultivars considered resistant to potato cyst nematode but not in
susceptible cultivars (Uehara et al., 2015). The gene has been considered to have
potential as a valuable source of resistance against potato cyst nematodes
(Ernst et al., 2002).
Resistant
varieties are based on
Solanum andigena, but continuous
cropping of resistant cultivars selects for
G. pallida
or pathotypes.
In 1966, plant breeders at Cornell University developed a new potato variety,
Peconic. Peconic is similar to Katahdin, a common cultivar in New York, and is
resistant to the race of G. rostochiensis that occurs in Newfoundland
(Spears, 1968). More recently, resistant cultivars have been
developed from S. tuberosum ssp andigena
and S. vernei germplasm that have resistance to both G. rostochiensis
and G. pallida (Brodie et al, 2000).
In Europe, many cultivars are available which are resistant to the potato
cyst nematodes.
Stabilizing selection by alternation of resistant and susceptible cultivars, and crop
rotation, are effective (Jones, 1969).
Crop Rotation and Cultural Control - When a non-host crop is grown,
population levels of the potato cyst nematodes are reduced, since each year a
certain proportion of the eggs within the cysts either die or hatch. After a
period of five to nine years, the population is reduced to a low level and a
successful host crop can be grown. Warmer climates permit greater rates of
annual decrease, thus reducing the interval between susceptible crops (Winslow
and Willis, 1972).
Soil Solarization - Soil solarization is essentially a mulching process
that involves the use of clear plastic film laid over moist soil. After several
months during periods of high air temperature, physical and biological changes
occur in the soil which can be beneficial to crops (Stapleton and DeVay, 1986).
Soil solarization has been used to control G. rostochiensis under New
York field conditions (LaMondia and Brodie, 1984). Populations were reduced by
96-99% within the top 10 cm of soil, and encysted juveniles were eradicated to a
depth of 5 cm. Since the central valley of California has higher air
temperatures and more solar radiation during the summer months than New York,
control of the potato cyst nematodes by soil solarization may be more effective
under California conditions (Pullman et. al., 1984).
Biological Antagonists - Nematodes, like other organisms, have natural
enemies which can reduce their ability to survive and reproduce. Fungi and
rickettsia-like micro-organisms have been investigated for their potential to
control the potato cyst nematodes (Jatala et al., 1979).
Additional Information and Resources
Australasian Plant Pathology Society Factsheets on Plant-parasitic Nematodes
(Prepared by Dr. Graham R. Stirling)
(Use your Return Key or click the Index Tab to return to this Nemaplex page)
-
PSN 053. Biosecurity measures to reduce the spread of Potato cyst
nematode in Australia
- Alvarez, M.G. 1972. Planning for the campaign against the golden nematode.
Guanajuato, Guanajuato: XX Mexican-American Plant Protection Work Conference.
- Behrens, E. (1975) Globodera Skarbilovic, 1959, eine selbstindige Gattung
in der Unterfamilie Heteroderinae Skarbilovic, 1947 (Nematoda: Heteroderidae).
Vortragstagung zu
aktuellen Problemen der Phytonematologie am 29/5/1975 in
Rostock. Manuskriptdruck der Vortrage. Rostock, 1975, pp.12-26.
- Brodie, B. B.; Scurrah, Maria; Plaisted, R. L.. 2000. Release of
germplasm resistant to multiple races of potato cyst nematodes. American Journal
of Potato Research 77: 207-209.
- Chitwood, B.C. 1951. "The golden nematode of potatoes." U.S.
Department of Agriculture Circular. No. 875.
- Chitwood, B.G., and E.M. Buhrer. 1945. "Summary of soil fumigant tests
made against the golden nematode of potatoes (Heterodera rostochiensis,
Wollenweber), 1942-1944." Proceedings of the Helminthological Society of
Washington. 12:39-41.
- Chizhov, V.N., Udalova, Z.V. and Nasonova, L.V. 2008. [Globodera
arenaria n.sp. and Cactodera radicale n.sp. (Nematoda: Tylenchida)
from rhizosphere of meadows in Mid-Volga region] russian Parasitological
Journal 2:109-116.
- CIH Descriptions of Plant-parasitic Nematodes, Set 2, No. 16 (1973).
- Clarke, A.J., and J. Hennessy. 1984. "Movement of Globodera
rostochiensis (Wollenweber) juveniles stimulated by potato-root exudate."
Nematologica. 30:206-212.
-
Devine, K.J. and
Jones, P.W. 2003. Comparison of the production and mobility of the potato cyst
nematodes, Globodera rostochiensis and G. pallida hatching
factors within a field planted with a host potato crop.
Nematology 5: 219-225.
- Ellis, P.R.
and Maxon-Smith, J.W. (1971) Inheritance of resistance to potato
cyst-eelworm (Heterodera rostochiensis Woll.) in the genus Lycopersicon.
Euphytica 20, 93-101.
- Endo, B.Y. 1971. "Nematode-induced syncytia (giant cells). Host-parasite
relationships of Heteroderidae." Pp. 91-117, B.M. Zuckerman, W.F. Mai, and
R.A. Rohde (eds.), Plant Parasitic Nematodes, Volume II. New York:
Academic Press.
- Ernst, K., Kumar, A., Kriseleit, D., Kloos, D-U, Phillips, M.S., Ganal,
M.W. 2002. The broad-spectrum potato cyst nematode resistance gene (Hero)
from tomato is the only member of a large gene family of NBS-LRR genes with
an unusual amino acid repeat in the LRR region. The Plant Journal
31:127-136.
- Evans, K., and B.B. Brodie. 1980. "The origin and distribution of the
golden nematode and its potential in the U.S.A." American Potato Journal.
57:79-89.
- Evans, K., and A.R. Stone. 1977. "A review of the distribution and
biology of the potato cyst-nematodes Globodera rostochiensis and G. pallida."
PANS. 23:178-189.
- Ferris, H. Teaching notes.
- Ferris, J.M. 1956. "Effect of soil temperature on the life cycle of the
golden nematode in host and nonhost species." Phytopathology.
47:221-230.
- Golden, A.M., and D.M.S. Ellington. 1972. "Redescription of Heterodera
rostochiensis (Nematoda: Heteroderidae) with a key and notes on closely
related species." Proceedings of the Helminthological Society of
Washington. 39:64-78.
- Goodey, J.B., and M.T. Franklin. 1958. Pp. 140, The Nematode Parasites of
Plants Catalogued under their Hosts. Farnham, England: Commonwealth
Agricultural Bureaux.
- Goodey, J.B., and M.T. Franklin. 1959. Pp. 66, Supplement to the Nematode
Parasites of Plants Catalogued under their Hosts, 1955-1958. Farnham,
England: Commonwealth Agricultural Bureaux.
- Green, C.D., D.N. Greet, and F.G.W. Jones. 1970. "The influence of
multiple mating on the reproduction and genetics of Heterodera rostochiensis
and H. schachtii." Nematologica. 16:309-326.
- Jatala, P., R. Kaltenbach, and M. Bocangel. 1979. "Biological control of
Meloidogyne incognita acrita and Globodera pallida on
potatoes." Journal of Nematology. 11:303.
- Knoetze, R., Malan, A.P., Mouton C. 2006. Differentiation of South African
potato cyst nematodes (PCN) by analysis of the rDNA internal transcribed
spacer region. African Plant Protection 12:103-110.
Kort, J.H., H.J. Ross, Rumpenhorst, and A.R. Stone. 1977. "An
international scheme for identifying and classifying pathotypes of potato
cyst-nematodes Globodera rostochiensis and G. pallida."
Nematologica. 23:333-339.
- LaMondia, J.A., and B.B. Brodie. 1984. "Control of Globodera
rostochiensis by solar heat." Plant Disease. 68:474-476.
- Luc, Maggenti & Fortuner, Rev. Nematol.
11(2):159-176 (1988)
- Mai, W.F. 1977. "Worldwide distribution of potato-cyst nematodes and
their importance in crop production." Journal of Nematology.
9:30-34.
- Mai, W.F., and B. Lear. 1953. "The golden nematode." Cornell
Extension Bulletin. 870.32 (Reprint No. 718).
- Matos, A. and M. Canto-Saenz. 1990?? Worldwide distribution of the
potato cyst nematode Globodera spp. International Potato Center.
P.O. Box 5969. Lima - Peru. (Published in Nematropica)
- Pullman, G.S., J.E. DeVay, C.L. Elmore, and W.H. Hart. 1984. "Soil
solarization, a nonchemical method for controlling diseases and pests." Cooperative
Extension, University of California, Division of Agriculture and Natural
Resources, Leaflet. 21377.
- Spears, J.F. 1968. "The golden nematode handbook: survey, laboratory,
control, and quarantine procedures." USDA Agriculture Handbook. No.
353.
- Stapleton, J.J., and J.E. DeVay. 1986. "Soil Solarization: a
non-chemical approach for management of plant pathogens and pests." Crop
Protection. 5:190-198.
- Stone, A.R. 1973a. "Heterodera pallida N. Sp. (Nematoda:
Heteroderidae), a second species of potato cyst nematode." Nematologica.
18:591-606.
- Stone, A.R. 1973b. "Heterodera pallida." Commonwealth
Institute of Helminthology Descriptions of Plant-Parasitic Nematodes, Set 2,
Nos. 16-17.
- Stone, A.R. 1973c. "Heterodera
rostochiensis." CommonwealthInstitute of Helminthology Descriptions of Plant-Parasitic Nematodes, Set 2,
Nos. 16.
-
Subbotin, S.A., Mundo-Ocampo, M., Baldwin, J.G. 2010.
Systematics of Cyst Nematodes (Nematode: Heteroderinae). Nematology Monographs
and Perspectives Volume 8A , D.J. Hunt and R.N. Perry (eds) Brill, Leiden, The
Netherlands 351p
- Trudgill, D.L., M.J. Elliott, K. Evans and M.S. Phillips. 2003. The white
potato cyst nematode (Globodera pallida) - a critical analysis of the
threat in Britain. Annals of Applied Biology
143:73-80.
-
Uehara, T.,
T. Narabu, K.
Ito, C.
Masuta. 2015.
Detection of the potato cyst nematode resistance gene Hero
A in
Japanese tomato cultivars by the PCR-RFLP method. Nematological Research
45:115-120.
- Varypatakis. K., Jones. J.T., Blok, V.C.
2019. Susceptibility of potato varieties to populations of Globodera pallida
selected for increased virulence. Nematology 21:995-998.
-
Wallace, H.R. 1964. Pp. 152-162,
The biology of plant parasitic nematodes.
New York: St. Martin's Press.
-
Whitworth, J.L., R.G. Novy, I.A. Zasada, X. Wang, L-M.
Dandurand, and J. C. Kuhl 2018. Resistance of Potato Breeding Clones and
Cultivars to Three Species of Potato Cyst Nematode. Plant Disease
102:2120-2128.
- Winslow, R.D., and R.J. Willis. 1972. "Nematode diseases of potatoes.
II. Potato cyst nematode, Heterodera rostochiensis." Pp.
18-34, J. Webster (ed.), Economic Nematology. New York: Academic Press.
Copyright © 1999 by Howard Ferris.
Revised:
January 15, 2026.