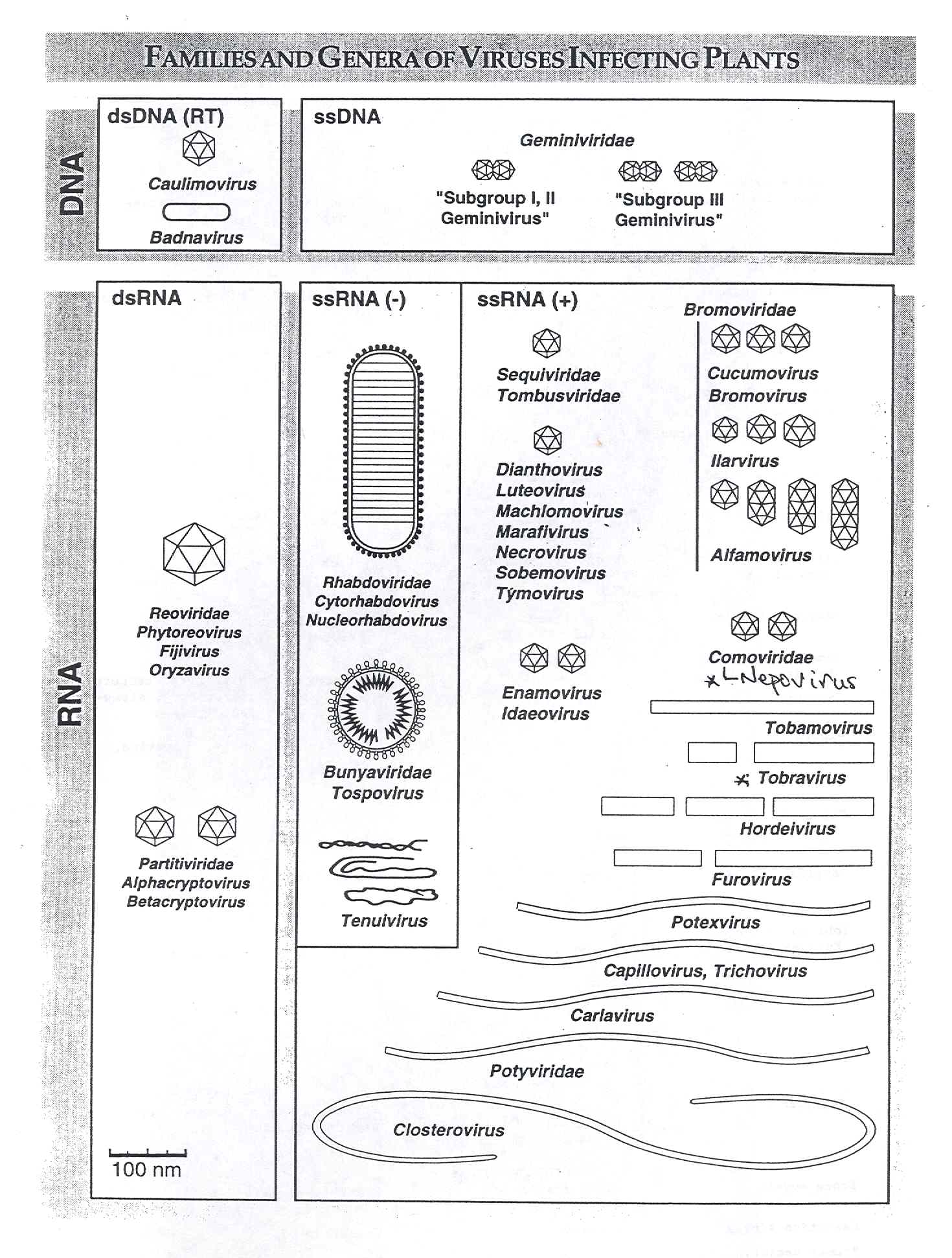
Rev. 10/30/19
Nepoviruses (Nematode-transmitted Polyhedral viruses) and Tobraviruses (Tobacco Rattle viruses) have nematode vectors (Lamberti and Roca, 1987).
Nepoviruses are isometric (polyhedral) particles around 30 nm in diameter (1 nm=10-9 m, 1 Ám=10-6 m). The only known nematode vectors are in the genera Xiphinema and Longidorus.
Tobraviruses are straight tubular particles with two size ranges, 180-210 nm and 45-115 nm. Trichodorus and Paratrichodorus are vectors.

The demonstration by Hewitt et al. (1958) that certain nematodes are vectors of plant viruses initiated research in Nematology and Virology that resulted in understanding of the transmission and etiology of an important group of soil-borne plant virus diseases.
11 spp of Xiphinema transmit 13 NEPO viruses
11 spp of Longidorus transmit 10 NEPO viruses
14 spp of Trichodorus transmit various strains of two TOBRA viruses:
tobacco rattle and pea early browning.
Lamberti (1987) suggests that since several trichodorid spp transmit the
same virus, and that both viruses are transmitted by the same nematode,
vector specificity is less developed in trichodorids than in longidorids.
Trichodorids may retain the virus for up to a year.
Acquisition time may be less than an hour to several days, depending on the
feeding characteristics of the nematode.
 |
| Retention sites: Longidorus
- odontostyle
area Xiphinema - odontophore and esophagus region Trichodorus - onchiostyle and esophagus. |
|
Selectively and specifically adsorbed at retention site - indicating a specific association between protein coat of virus and cuticular surface. Dissociation with cuticle probably occurs as glandular secretions pass forward into the plant cell. |
In Xiphinema and Trichodorus, although the lining of the esophagus is not shed at a molt, it undergoes structural changes and virus particles may pass into the intestine. In Longidorus the stoma, odontostyle and guiding sheath are shed.
X. americanum (sensu lato) transmits tobacco ringspot and
tomato ringspot.
The virus is acquired within 24 hours of the initiation of
feeding.
Virus particles are transmitted by both adults and juveniles.
X. americanum transmits the tomato ringspot virus strains:
peach rosette mosaic virus
peach yellow bud mosaic virus
cherry rasp leaf virus
grapevine yellowvein virus
Particles are carried in the esophagus lumen.
Lamberti et al. consider that X. americanum is not a vector of tomato ringspot virus, although it is widely reported to be. They contend
that the reports represent a mis-identification especially in California where they feel that the vector is X. californicum. However, Griesbach
and Maggenti (1989) found a wide range of vectoring capabilities of tomato ringspot viruses with California populations of X. americanum sensu lato.
On the basis of considerable overlap of morphological and morphometric characters among putative species, they synonymized X. americanum and
X. californicum (Griesbach and Maggenti (1990). That synonymy was later rejected (Robbins and Brown, 1991). The situation is not yet clearly
resolved; studies on developmental biology and the application of molecular techniques are providing further information on the diversity
within the whole X. americanum group (Halbrendt and Brown, 1993; Vrain,1993).
Lamberti suggests that the same mis-identification occurs with the vector of Cherry Rasp Leaf virus in California.
There is a need for the application of molecular biology techniques in this problem of diagnostics.
X. californicum transmits prune brown line and Prunus
stem pitting strains of tomato ringspot virus readily and the cherry leaf mottle
strain rarely. (Hoy, Ph.D thesis, UC. Davis)
X. diversicaudatum transmits Arabis mosaic virus to
variety of crops.
Adults retain the virus for at least 8 months.
X. index - grapevine fanleaf virus can be acquired in 5 to 15
minutes, persists up to 9 months when nematode not feeding.
Longidorus elongatus transmits raspberry ringspot and tomato
blackring
viruses.
Longidorus leptocephalus, L. attenuatus, L. elongatus, Trichodorus spp and viruses are involved in Docking disorder of sugarbeets (named for Docking
region in south of England). Stunted growth in spring due to nematodes, virus symptoms in foliage. Effects are most pronounced in spring, heavy
rainfall in May seems to increase the problem. In July the affected plants start to grow again and may achieve almost normal foliage, but a much
reduced tap root. The severity of Docking disorder varies from year to year with the climate.
Management by rotation is difficult as several nematode species are involved, each with a differing host range. Also host ranges of these
nematodes are not completely known.
1,3-D nematicide applied in the plant row prior to planting reduces the nematode populations and increases yield, but only in well-drained alkaline
soils. The treatment is not always effective at economically feasible rates.
Grapevine Fanleaf Virus Degeneration is the oldest known disease of Vitis vinifera.
Records in Europe date back 200 years and specimens in herbaria displaying symptoms predate the introduction of American rootstocks (Martelli and Sovino, 1988). Grapevine Fanleaf Virus Degeneration is the oldest known disease of V. vinifera.
The observation that X. index transmits grapevine fanleaf virus (GFLV) was the first record of virus transmission by a nematode (Hewitt et al., 1958).
The virus is lost during the molt of the cuticle between life stages. The virus is not passed through the egg stage. Consequently, the virus is re-acquired by feeding of each vermiform life stage of the nematode. GFLV causes reduced vigor, lack of fruit set, and reduced yield of grapevines. Of great importance in the design of control programs for the X. index / GFLV problem is that portions of the grape root system can remain alive and serve as a reservoir for virus and nematode for at least 5 years after vine trunks have been removed (Raski et al., 1965).
The grapevine fanleaf virus is a Nepovirus with isometric particles of 30nm diameter. It is transmitted by X. index and X. italiae, with X. index the more efficient vector (Argelis, 1987; Martelli and Savino (1988).
The virus can be acquired in less than 15 minutes of feeding on an infected plant and is retained up to eight months in the absence of feeding (Taylor and Robertson, 1975).).
The retention site for GFLV in X. index is the cuticular lining of the esophagus, including the area surrounding the odontophore (Taylor and Robertson, 1975). As with other viruses vectored by longidorid and trichodorid nematodes, it is selectively and specifically adsorbed at the retention site, indicating a specific association between protein coat of virus and cuticular surface. Dissociation with cuticle probably occurs as glandular secretions pass forward into the plant cell (Taylor and Robertson, 1975).
The virus does not pass through the egg stage. It is not retained through a molt when the esophageal lining and odontostyle are also shed. In Xiphinema, although the lining of the esophagus is not shed at a molt, it undergoes structural changes and virus particles may pass into the intestine (Taylor and Robertson, 1975).
The virus does not replicate in the nematode vector.
Detection:
From a management standpoint, it is important to determine whether X. index is present in a site intended for a vineyard, and whether the nematodes are viruliferous.
Virus particles can be detected in extracts from single nematodes by immunosorbent electron microscopy (Roberts and Brown, 1980), however, as indicated by Esmenjaud et al (1993) the procedure is complex and not readily adapted to routine assays.
Enzyme-linked immunosorbent assay (ELISA) methods are routinely used to determine GVFL in plant tissue (Walker et al, 1994). When applied to nematodes, the techniques appear sufficiently sensitive to detect the virus if more than ten viruliferous individuals are used, but are unreliable for single individuals (Esmenjaud et al, 1993).
Symptoms:
Martelli and Savino (1988) describe three distinct syndromes of symptoms of grapevine fanleaf degeneration:
1. Malformations: Leaves are variously distorted, asymmetrical, with wide petiole sinuses and abnormal vein arrangement that results in the appearance of an open fan. Leaf symptoms may also include chlorotic mottling. Foliar symptoms are most evident in the spring and through the vegetative growth period. Shoots show abnormal branching, with double nodes and short internodes, while forming angles at internodes. Bunches are small with poor fruit set and shot berries. They ripen irregularly.
2. Yellow mosaic: Bright yellow discoloration of leaves, shoots, tendrils and inflorescences in the spring. Foliage and clusters are not malformed, but clusters are small.
3. Veinbanding: Yellow flecking along main veins and associated interveinal areas of mature leaves appearing mid to late summer. Leaves are not malformed, but fruit set is poor and yield is low.
Several closely related nepoviruses found in grapevines are apparently related to GFLV but are serologically different. They are sap-transmissible, and where vectors have been definitively established they are species of Xiphinema and Longidorus. They include arabis mosaic virus, raspberry ringspot virus, tomato blackring virus, grapevine chrome mosaic virus, Strawberry latent ringspot virus, artichoke Italian latent virus, and grapevine Bulgarian latent virus (Argelis, 1987; Stellmach and Goheen, 1988).
One or more of ten species of Xiphinema are present in all major grape-growing regions of the world. They are X. algeriense, X. americanum, X. brevicolle, X. diversicaudatum, X. index, X. italiae, X. mediterraneum, X. pachtaicum, X. turcicum, and X. vuittenezi (Raski, 1988). With X. index a notable exception, most species of Xiphinema have a wide host range and are adapted to a wide range of soil textures.
Xiphinema americanum is actually considered a group of some 39 species, which include X. brevicolle and X. pachtaicum. Molecular techniques (Vrain, 1993), biological evaluations (Halbrendt and Brown, 1993) and virus transmission studies (Brown et al., 1993) are continuing to underscore the variability and similarities within the X. americanum group. These studies are adding to the body of knowledge that will be required for an eventual taxonomic revision. The group also includes X. californicum Lamberti and Bleve-Zacheo, which is probably associated with grape in California (Lamberti and Ciancio, 1993).
Tomato Ringspot Virus in Grapevines
Tomato Ringspot Virus Degeneration is also called grape yellow vein disease, tomato ringspot disease, and little berry disease. It is endemic in the northeastern U.S. and Canada, and occurs less frequently in California (Gonsalves, 1988). The nepovirus is transmitted by X. americanum, X. californicum, and X. rivesi. Particles are carried in the esophagus lumen adhering to the cuticular lining. Since there is considerable variability in the species complex that is loosely-termed X. americanum, Lamberti and Bleve-Zacheo (1979) contend that reports of X. americanum as a vector of TRSV represent a misidentification, or predate taxonomic revisions. They consider that X. californicum is the vector of TRSV in California. However, Griesbach and Maggenti (1989) found a wide range of vectoring capabilities of TRSV strains with California populations of X. americanum sensu lato.
Symptoms of Tomato Ringspot Virus Degeneration differ geographically, with the disease more severe in colder regions. Leaves are mottled, with oak leaf patterns, new growth is weak and buds are susceptible to cold damage. Internodes are shortened, leaf area is reduced, and clusters are small. The grape yellow vein symptom, common in infections with this virus in California, consists of yellow flecking along the veins and associated lamina. There is reduced fruit set and slow decline of the vine (Gonsalves, 1988).
Peach Rosette Mosaic Virus in Grapevines
Peach Rosette Mosaic Virus Decline is a disease of V. labrusca L. grapevines that occurs only in Michigan in the U.S. The virus also causes a disease in peach, as indicated by the name (Ramsdell, 1988). This nepovirus is a strain of TRSV transmitted by X. americanum and Longidorus diadecturus with the characteristics of other nepoviruses transmitted by these genera. The virus may be endemic in vineyard weeds, which complicates exclusionary measures. Symptoms of Peach Rosette Mosaic Virus Decline include puckered leaves with flattened basal sinuses, sparse clusters, and crooked shoots. The vines are susceptible to winter injury (Ramsdell, 1988).
Management Guidelines for GVFLV - Pre-plant
AVOIDANCE
The wide host ranges of ectoparasitic nematodes make pest avoidance difficult to achieve. One exception is X. index and GFLV. The use of virus-free and nematode-free rootings provide the best method of controlling the complex and certification programs have reduced its dissemination through vegetative propagation (Esmenjaud et al., 1993). The wide host range and current abundance of X. americanum in U.S. soils is an indication that quarantine of the nematode is not possible, but the certified virus-free nursery stock program continues to have merit. On-farm nurseries should be maintained nematode-free and located away from old vineyard sites.
CULTURAL CONTROLS
By their nature, ectoparasitic nematodes can survive in soil or persist on weed hosts for long periods of time. Fallowing will only reduce populations, not remove them. The persistence of old grape roots in replant sites is a problem. New grape roots tend to follow the channels in the soil left by old roots. GFLV can be moved from these reservoirs to healthy planting stock through exploratory feeding probes by X. index. It may be difficult to develop 'immune' rootstocks on which the nematode cannot reproduce and never even attempts to feed. Replanting of a virus-infected vineyard with a rootstock that is resistant to X. index within 6-7 years after removing a virus infected vineyard will not be successful in the long term unless the nematode does not attempt to feed. The site will probably not be safe until 1-2 years after the last old root is dead. Such time intervals may not be economically feasible. Resistance to GFLV will be an important solution to the problem (Esmenjaud, 1986).
Old roots are most easily killed before the old vine is removed by using systemic herbicides. Once the vine is removed the only reliable method of killing roots in the top 2 m of soil is by soil fumigation. Soil ripping to that depth on 30 or 60 cm centers may increase the speed of root death, but this tactic has not been field tested. Heat is a useful root killing agent but we are unable to deliver the required heat using current methods. Soil flooding for months will not kill old grape roots or nematodes. Selected rotation crops will reduce population levels of certain nematode species over time. This will have little value for endoparasites but should be tested with ectoparasites.
Certain Sorghum bicolor L. x Sorghum sudanense Stapf hybrids grown in summer have negative effects on ectoparasites but the number of years required to eliminate a nematode problem is unknown. Addition of manure, composts and ammonia does not kill old roots, but there are various reports of the benefits of ammonia as a nematode control agent (Mojtahedi and Lownsbery, 1976).
CHEMICAL CONTROLS
Soil fumigation with methyl bromide or 1,3-Dichloropropene is effective for killing old roots 1.5 and 2 m deep in soil. Such treatments can also give 99.9% reduction of all nematode species in the top 1.5 to 2 m of soil when properly applied. Two to six years after such treatments, the nematodes do return unless resistant rootstocks are replanted. The use of high rates of 1,3-dichloropropene was halted in the state of California in spring 1990. Methyl bromide use is to be phased out by the year 2000, and adequate replacement fumigants must be environmentally safe. Environmental problems with nematicides have also occurred elsewhere (Rupp, 1990).
The future of all general biocides as a fumigation replacement is unclear. An approach we have taken is the integration of "softer" root killing agents or tools coupled with "softer" soil treatments which reduce soil-dwelling population levels. The practice of removing a vineyard and replanting within one year is not a current option in vineyard management where nematode problems occur. There will be much discovery in this area in the decades ahead. One treatment strategy worthy of study involves use of root killing and soil cleansing treatments followed by one or more years of non-host crops.
Management Guidelines for GVFLV - Established Vineyards
AVOIDANCE
Movement of contaminated grape harvesting equipment and tractors from a site infected with X. index should be avoided.
BIOLOGICAL CONTROL
Refer to the previous chapter on this subject however, the addition of biological agents to soil has been inconsistent and usually ineffective. It may be possible, however, with the advent of drip irrigation systems, to apply microbe-produced toxins directly to the soil in irrigation water. Zoosporic fungal parasites have been isolated from X. rivesi and X. americanum. Such fungi require free water for their movement through soil. Their occurrence suggests research to investigate water management protocols that will enhance fungal parasitism of dagger nematodes.
CULTURAL CONTROLS
By reducing vine stress through more frequent irrigation the damage caused by nematodes can be reduced.
The use of grassy cover crops in vineyards infested with X. index should be studied. However, legume cover crops in vineyards should be monitored to assess population levels of Mesocriconema xenoplax, which may increase. Populations of X. americanum will also increase on most cover crop selections. The difficulty in choice of cover crops is that the host range of most ectoparasites is quite broad.
Nematode species with long bodies tend to be in shallow and non-disturbed sites of a vineyard, so placement of any treatment is important. Tillage and soil disturbance can reduce population levels short periods of time, but root surface area is also reduced.
Drip-irrigation-applied fertilizers that release ammonia may reduce population levels of ectoparasitic nematodes when applied repeatedly. Fifteen kg/ha of nitrogen in urea salt when re-applied three to five times at 30 to 45 day intervals can reduce population levels of most ectoparasites by half. More field testing of these strategies is necessary. Since grapevines do not have a high nitrogen requirement and some vineyards already have excess nitrogen, this technique should be tested and implemented with caution.
CHEMICAL CONTROLS
Organophosphate and carbamate nematicides are lethal to ectoparasitic nematodes when used as single treatments at high rates via drip irrigation. Treatments with currently-available commercial nematicides only reduce populations about 50% for 6 to 8 months after treatment. Multiple treatments with low rates of phenamiphos (1 kg/ha) are ineffective against ectoparasites. When multiple treatments are used against endoparasites for several years, population levels of ectoparasites such as X. americanum can be observed to increase above the non-treated population levels.
In the case of ectoparasites, the value of systemic nematicides will be minimal unless the toxicant is available during feeding or leaks out into the rhizosphere (Edwards, 1991).
Future Research
Except for X. index, ectoparasitic nematodes have not received the same research attention as endoparasites. Long-term experiments on the damage potential and management of ectoparasitic nematodes in vineyards are needed.
The apparent increase in incidence of X. index associated with reduced use of conventional soil fumigants is important and should be monitored. Other problems with ectoparasitic nematodes may similarly emerge. New pre-plant control strategies will be needed in the near future. Resistance and tolerance of rootstocks will continue to be important areas of study.
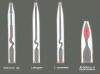
Trichodorus viruliferous - transmits tobacco rattle and pea early browning virus. It is sometomes involved in Docking disorder in sugarbeets in England. Trichodorus similis - transmits tobacco rattle virus. At least 14 species of Trichodoridae have been demonstrated to vector Tobra viruses.
Tobacco rattle virus (genus Tobravirus) is a multi-component virus with rod-shaped long (RNA-1) and short (RNA-2).particles (Mojtahedi et al., 2000).
M-type (normal) viral isolates contain both RNA-1 and RNA-2; they code for coat protein synthesis and are readily transmitted mechanically and by trichodorid nematodes (Stevenson et al., 2001)..
NM-type viral isolates have only contain RNA-1. They can be sap-transmitted and move through the plant systemically. Since they do not code for coat protein synthesis are not transmitted by trichodorid nematodes (Dale et al., 2004).
The multi-component nature of the particles results in considerable variation among isolates (Brown et al., 2000; Mojtahedi et al., 2001).
Corky ringspot is caused by tobacco rattle virus (Tobravirus), which is vectored by stubby-root nematodes (Paratrichodorus spp. and Trichodorus spp.). The condition is sometimes referred to as Spraing, a Scottish word meaning a bright streak or stripe (de Bokx, 1972).
Corky ringspot symptoms vary depending on virus strain, potato cultivar, and time of infection. Symptoms often include of brown necrotic rings, arcs, and diffuse spots which are considered quality defects and may result in after-harvest devaluation or rejection of either table or processed potatoes.
 |
 |
| External Symptoms of Corky Ringspot Virus on a Potato Tuber | Internal Symptoms of Corky Ringspot Virus in a Potato Tuber |
Corky ringspot symptoms in potato include necrotic rings and pits on the tuber surface and range from diffuse brown spots to concentric rings or arcs of brown, necrotic tissue to dark-brown necrotic tissue which extends through tuber flesh (Mojtahedi et al., 2001). The virus is usually detectable when symptoms are seen but may also be present in asymptomatic tissue (Charlton, 2006).
Tubers from soil with a history of tobacco rattle virus serve as a reservoir. The virus may spread to daughter tubers when infected tubers are used as seed (Crosslin et al., 1999). Newly formed potato tubers are quite vulnerable to tobacco rattle virus infection and tubers as small as 3-cm in diameter had corky ringspot blemishes in tobacco-rattle infested fields in Florida (Weingartner et al. (1975).
Tobacco Rattle Virus is reported in many areas of the world and more than 400 plant species in 50 plant families are susceptible to infection (Brunt et al., 1996; Dallwitz, 1980; Dallwitz et al., 1993; Hooker, 1981; Ploeg et al., 1989).
Several species of Trichodorus and Paratrichodorus transmit M-type isolates of Tobacco Rattle Virus (Harrison and Robinson, 1986). Stubby-root nematodes are migratory ectoparasites that are mobile during each stage of their life cycle (Stark and Love, 2003) and feed primarily on meristematic cells or root tips which hinders root elongation (Crow, 2005). Damaged root tips may swell and lateral roots may emerge behind them, resulting in root proliferation.
The life cycle of stubby-root nematodes is not well studied.
Eggs of
Paratrichodorus
In general, Trichodoridae nematodes prefer coarser textured soils and avoid desiccation stress by migrating vertically to moister soil layers. Vertical movement of Trichodoridae nematodes appears greatest when soil pores are half-full of water and least in waterlogged or dry soil conditions (Decraemer, 1995). Since soil moisture is often maintained below field capacity for optimum yield and quality in potato production, vertical migration may explain the difficulty of consistently finding stubby-root nematodes during routine soil sampling procedures to 30-cm depth in Washington and Oregon (Charlton, 2006).
Stubby-root feeding injury is not important economically and is rarely visible in potato, however, corky ringspot virus can be transmitted at very low population densities of the nematode (Charlton, 2006).
The wide host range for both virus and vectors make eradication of corky ringspot in potato fields difficult. Alfalfa (Medicago sativa), a rotation crop in Oregon and Washington, and spearmint (Mentha cardiaca) do not appear to serve as hosts of tobacco rattle virus (Mojtahedi et al., 2002). Since the virus is lost each time the nematode molts, and since it does not pass through the nematode egg stage, P. allius are cleansed of tobacco rattle virus after being reared on these plants for a period of 1 to 3 months (Boydston et al. 2004). Therefore, it may be possible to eliminate tobacco rattle virus from populations of P. allius rotation crops that do not harbor the virus. Of course, the crops must be kept free of weed hosts for tobacco rattle for the strategy to be effective (Charlton, 2006)
Aldicarb (Temik 15G), a systemic carbamate pesticide, suppresses corky ringspot (damage in potato by controlling stubby-root nematodes (Weingartner and Shumaker, 1990; Rykbost et al., 1992).
1,3-dichloropropene
(Telone II) was effective in reducing
corky ringspot in Washington but not in Florida or the Klamath Basin of Oregon
(Ingham et al., 2000; Weingartner et al., 1990;
Rykbost et al., 1995). The fumigation failure in
Metam sodium (Vapam HL) delivered through chemigation (water-run) is not effective against corky ringspot of potatoes when used alone (Ingham et al., 2000).
Return to Plant Parasites Menu
References
Al Banna, L. and Gardner, S. L. (1993). Three new species of nematodes
associated with endemic grape (Vitis) in
Argelis, A. (1987). Present situation of grapevine virus diseases with
reference to the problems which they cause in Greek vineyards. Pp 309-312 in
Integrated
Ayala, A., M.W. Allen, and E.M.
Noffsinger. 1970. Host range, biology, and factors affecting survival and
reproduction of the stubby root nematode. J. Agr. Univ.
Boydston, R.A., H. Mojtahedi,
J.M. Crosslin, P.E. Thomas, T. Anderson, and
Brown, C.R., H. Mojtahedi, G.S.
Santo, P. Hamm, J.J. Pavek, D. Corsini, S. Love, J.M. Crosslin, and P.E. Thomas.
2000. Potato germplasm resistant to corky ringspot disease. American Journal of
Potato Research 77:23-27.
Brown, D. J. F. and Boag, B. (1975). Longidurus macrosoma. Commonwealth
Institute of Helminthology, Descriptions of Plant-parasitic Nematodes. No. 67.
Brown, D. J. F., Halbrendt, J. M, Robbins, R. T. and Vrain, T. C. (1993). Transmission of nepoviruses by Xiphinema americanum-group nematodes. Journal of Nematology 25, 349-354.
Chang, H. Y. and Raski, D. J. (1972). Hemicriconemoides chitwoodi on grapevines. Plant Disease Reporter 56, 1028-1030.
Charlton, B.A.
2006. Effects of oxamyl on suppression of the Tobacco rattle virus vector
Paratrichodorus allius and corky ringspot disease of potato in the
Charlton, B.A.
2006. Effects of oxamyl on suppression of the Tobacco rattle virus vector
Paratrichodorus allius and corky ringspot disease of potato in the
Cohn, E. and Mordechai, M. (1969). Investigations on the life cycles and host preference of some species ov Xiphinema and Longidorus under controlled conditions. Nematologica 15, 295-302.
Crosslin, J.M., P.E. Thomas, and
C.R. Brown. 1999. Distribution of tobacco rattle virus in tubers of resistant
and susceptible potatoes and systemic movement of virus into daughter plants.
American Journal of Potato Research 76:191-197.
Crow, W.T. 2005. Diagnosis of
Trichodorus obtusus and
Paratrichodorus minor on turfgrasses in
the
Dale, M.F.B, D.J. Robinson, and
D. Todd. 2004. Effects of systemic infections with Tobacco rattle virus on
agronomic and quality traits of a range of potato cultivars. Plant Pathology
53:788-793.
Dallwitz, M. J., T.A. Paine, and
E.J. Zurcher. 1993. User's Guide to the DELTA System: a general system for
processing taxonomic descriptions. 4th edition. 136 pp. (CSIRO Division of
Entomology:
Dallwitz, M.J. 1980. A general
system for coding taxonomic descriptions.
Taxon 29:41-46.
de Bokx, J.A. (Ed.). 1972.
Viruses of potatoes and seed-potato production. Centre for Agricultural
Publishing and Documentation, Wageningen.
Decraemer, W. 1995. The Family
Trichodoridae: Stubby root and virus vector nematodes. Kluwer Academic Publ.,
Edwards, M. (1991). Control of plant parasitic nematodes in sultana grapevines (Vitis vinifera) using systemic nematicides. Australian Journal of Experimental Agriculture 31, 579-584.
Esmenjaud, D. 1986. Les nematodes de la vigne. Phytoma 374, 24-27.
Esmenjaud, D., Walter, B., Minot, J. C., Voisin, R. and Cornuet, P. (1993). Biotin-avidin ELISA detection of grapevine fanleaf virus in the vector Xiphinema index. Journal of Nematology 25, 401-405.
Esmenjaud, D., Walter, B., Valentin, G., Guo, Z. T. and Cluzeau, D. (1992).
Vertical distribution and infectious potential of Xiphinema index
(Thorne and
Allen, 1950) (Nematoda:
Longidoridae) in fields affected by grapevine fanleaf virus in vineyards in the
Champagne region of
Ferris, H. and McKenry, M. V.
(1974). Seasonal fluctuations in the spatial distribution of nematode
populations in a
Ferris, H. and McKenry, M. V. (1975). Relationship of grapevine yield and growth to nematode densities. Journal of Nematology 7, 295-304.
Ferris, H., Lau, S. and Venette, R. C. (1994). Population energetics of bacterial-feeding nematodes: respiration and metabolic rates based on carbon dioxide production. Soil Biology and Biochemistry, in press.
Ferris, H., McKenry, M. V. and
Fortuner, R. (1987). A reappraisal of Tylenchina (Nemata). 8. The family Hoplolaimidae Filip'ev, 1934. Revue de Nematologie 10, 219-232.
Garcia Gil de Bernabe, A.
(1976). La Degeneracion Infecciosa y Las Enfermedades de Virus de la Vina En
La Zona Del Jerez.
Ministerio de Agricultura,
Madrid. 225 p.
Gonsalves, D. (1988).
Tomato ringspot virus decline. Pp 49-50 in Compendium of Grape
Diseases (R. C. Pearson and A. C. Goheen, Eds). American Phytopathological
Society Press,
Griesbach, J. A. and Maggenti, A. R. (1989). Vector capability of
Xiphinema americanum sensu lato in
Halbrendt, J. M. and Brown, D. J. F. (1992). Morphometric evidence for three juvenile stages in some species of Xiphinema americanum sensu lato. Journal of Nematology 24, 305-309.
Halbrendt, J. M. and Brown, D. J. F. (1993). Aspects of biology and development of Xiphinema americanum and related species. Journal of Nematology 25, 355-360.
Harrison, B.D., and D.J.
Robinson. 1986. Tobraviruses. Pages 339-369
In: The Plant Viruses Vol. 2: The
Rod-shaped Plant Viruses. M.H.V. van Regenmortel, and H. Fraenkel-Conrat, eds.
Plenum Press, N.Y.
Hewitt, W. B., Raski, D. J. and Goheen, A. C. (1958). Nematode vector of soil-borne fanleaf virus of grapevines. Phytopathology 48, 586-595.
Hooker, W.G. (Ed.). 1981.
Compendium of potato disease. American Phytopathological Society,
Ingham, R.E., P.B. Hamm, R.E.
Williams, and W.H. Swanson. 2000. Control of
Paratrichodorus allius and corky ringspot
disease of potato in the Columbia Basin of Oregon. Supplement of the Journal of
Nematology 32 (4S):566-575.
Jaffee, B. A. (1986). Parasitism of Xiphinema rivesi and X. americanum by zoosporic fungi. Journal of Nematology 18, 87-93.
Jaffee, B. A. and McInnis, T. (1991). Sampling strategies for detection of density-dependent parasitism of soil-borne nematodes by nematophagous fungi. Revue de Nematologie 14, 147-150.
Jenkins, W. R. (1964). A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Disease Reporter 48, 692.
Jensen, H.J., and
T.C. Allen. 1964.
Trichodorus allius, a potential nematode
vector of TRV. Phytopathology 54:1434.
Kirkpatrick, J. D., Van Gundy, S. D. and Martin, J. P. (1965). Effects of Xiphinema index on growth and abscission in Carignane grape, Vitis vinifera. Nematologica 11, 41.
Lamberti, F. and Bleve-Zacheo, T. (1979). Studies on Xiphinema americanum sensu lato with descriptions of fifteen new species (Nematoda: Longidoridae). Nematologia Mediterranea 7, 51-106.
Lamberti, F. and Ciancio, A. (1993). Diversity of Xiphinema americanum-group species and hierarchical cluster analysis of morphometrics. Journal of Nematology 25, 332-343.
Lamberti, F. and
Lider, L. A., Olmo, H. P. and Goheen, A. C. (1988). Hybrid Grapevine
Rootstock Patent No. 6166
Martelli, G. P. and Savino, V. (1988). Fanleaf degeneration. Pp 48-49 in
Compendium of Grape Diseases (R. C. Pearson and A. C. Goheen, Eds).
American Phytopathological Society Press,
McCarthy M. G. and Cirami, R. M. (1990). The effect of rootstocks on the
performance of Chardonnay from a nematode-infested
McKenry, M. V. (1992). Nematodes. Pp 279-293 in Grape Pest
Management (D. L. Flaherty, L. P. Christensen, W. T. Lanini, J. J. Marois,
P. A. Phillips and L. T. Wilson, Eds).
McKenry, M. V. and Kretsch, J. O. (1994). Interactions of selected Vitis rootstocks with ectoparasitic nematodes. American Journal of Enology and Viticulture, in press.
McKenry, M. V., Viveros, M. and Teviotdale, B. (1990). Criconema mutabile
associated with bacterial canker and Nemaguard rootstock. Plant Disease
74, 394.
Melakeberhan, H. and Ferris, H. (1988). Growth and energy demand of Meloidogyne incognita on susceptible and resistant Vitis vinifera cultivars. Journal of Nematology 20, 545-554.
Mojtahedi, H. and Lownsbery, B. F. (1976). The effects of ammonia-generating fertilizer on Criconemoides xenoplax in pot cultures. Journal of Nematology 8, 306-309.
Mojtahedi, H., G.S Santo, J.M.
Crosslin, C.R. Brown, and P.E. Thomas. 2000. Corky ringspot disease: Review of
the current situation. Proc. 39th
Mojtahedi, H., R.A. Boydston,
P.E. Thomas, J.M Crosslin, and R.A. Boydston. 2002. Eliminating tobacco rattle
virus from viruliferous
Paratrichodorus allius and establishing a
new virus-vector combination. Journal of Nematology 34:66-69.
Nyczespir, A. and Wood, B. W. (1988). Peach leaf senescence delayed by Criconemella xenoplax. Journal of Nematology 20, 585-589.
Orton Williams, K. J. (1972). Macroposthonia xenoplax. Commonwealth
Institute of Helminthology, Descriptions of Plant-parasitic Nematodes. No. 12.
Pinochet, J. and Raski, D. J. (1975). Four new species of the genus Hemicriconemoides (Nematoda: Criconematidae). Journal of Nematology 7, 263-270.
Pinochet, J., Raski, D. J. and Jones, N. O. (1976). Effect of Helicotylenchus pseudorobustus on 'Thompson Seedless' grape. Plant Disease Reporter 60, 528-529.
Radewald, J. D. and raski, D. J. 1962. A study of the lifecycle of Xiphinema index. Phytopathology 52, 748.
Ramsdell, D. C. (1988). Peach rosette mosaic virus decline. Pp 51-52 in
Compendium of Grape Diseases (R. C. Pearson and A. C. Goheen, Eds).
American Phytopathological Society Press,
Raski, D. J. (1952). On the morphology of Criconemoides Taylor 1936, with descriptions of six new species. Proceedings of the Helminthological Society of Washington 19, 85-99.
Raski, D. J. (1975). Revision of the genus Paratylenchus Micoletzky, 1922, and descriptions of new species. Part II of three parts. Journal of Nematology 7, 274-295.
Raski, D. J. (1988). Dagger and needle nematodes. Pp 56-59 in Compendium of Grape Diseases (R. C. Pearson and A. C. Goheen, Eds). American Phytopathological Society Press, St Paul, Minnesota. 93 p.
Raski, D. J. and Luc, M. (1987). A reappraisal of Tylenchina (Nemata). 10. The superfamily Criconematoidea Taylor, 1936. Revue de Nematologie 10, 409-444.
Raski, D. J. and Radewald, J. D. (1958). Reproduction and symptomatology of certain ectoparasitic nematodes on roots of Thompson Seedless grape. Plant Disease Reporter 42, 941-943.
Raski, D. J., Hewitt, W. B., Goheen, A. C., Taylor, C. E. and Taylor, R. H. 1965. Survival of Xiphinema index and reservoirs of fanleaf virus in fallowed vineyard soil. Nematologica 11, 349-352.
Robbins, R. T. and Brown, D. J. F. (1991). Comments on the taxonomy, occurrence and distribution of Longidoridae (Nematoda) in North America. Nematologica 37, 395-419.
Roberts, I. M. and Brown, D. J. F. (1980). Detection of six nepoviruses in their nematode vectors by immunosorbent electron microscopy. Annals of Applied Biology 96, 187-192.
Rupp, D. (1990). The leaching of nitrate and nematicide from trenched vineyards. Wein-Wissenschaft 45, 135-140.
Rykbost, K.A., R.E. Ingham, and
J. Maxwell. 1992. Control of nematodes and related disease in potatoes.
In: Research in the
Rykbost, K.A., R.E. Ingham, and
J. Maxwell. 1995. Control of nematodes and related disease in potatoes.
In: Research in the
Santo, G. S. and Bolander, W. J. (1977). Effect of Macroposthonia xenoplax on the growth of concord grape. Journal of Nematology 9, 215-217.
Schneider, S.M., and H. Ferris.
1987. Stage-specific population development and fecundity of
Paratrichodorus minor. Journal of
Nematology 19:267-394. Stark, J.C., and S.L. Love (Eds.). 2003. Potato
Production Systems.
Seshadri, A. R. (1965). Investigations on the biology and life cycle of Criconemella xenoplax Raski, 1952 (Nematoda: Criconematidae). Nematologica 11, 540-562.
Siddiqui, I. A., Sher, S. A. and French, A. M. 1973. Distribution of Plant Parasitic Nematodes in California California Department of Food and Agriculture, Sacramento, California. 324 p.
Stellmach, G. and Goheen, A. C. (1988). Other virus and virus-like diseases.
Pp 53-54 in Compendium of Grape Diseases (R. C. Pearson and A. C.
Goheen, Eds). American Phytopathological Society Press,
Stevenson, W.R., R. Loria, G.D.
Franc, D.P. Weingartner (Eds.) 2001. Compendium of Potato Diseases 2nd
Edition. American Phytopathological Society,
Taylor C. E. and Robertson, W. M. (1975). Acquisition, retention and
transmission of viruses by nematodes. Pp 253-276 in Nematode Vectors of Plant
Viruses (F. Lamberti, C. E. Taylor and J. W. Seinhorst, Eds). Plenum Press,
Thomas, H. A. (1959). On Criconemoides xenoplax Raski with
special reference to its biology under laboratory conditions. Proceedings of
the Helminthological Society of
Timper, P. and H. K. Kaya. (1989). Role of the second-stage cuticle of entomogenous nematodes in preventing infection by nematophagous fungi. Journal of Invertebrate Pathology 54, 314-321.
Van Gundy, S. D. and Rackham, R. L. (1961). Studies on the biology and pathogenicity of Hemicycliophora arenaria. Phytopathology 51, 393-397.
Vrain, T. C. (1993). Restriction fragment length polymorphism separates species of the Xiphinema americanum group. Journal of Nematology 25, 361-364.
Walker, M. A., Wolpert, J. A. and Weber, E. (1994). Field screening of grape rootstock selections for resistance to fanleaf degeneration. Plant Disease, in press.
Weingartner, D.P., and J.R.
Shumaker. 1990. Effects of soil fumigants and aldicarb on corky ringspot disease
and trichodorid nematodes in potato. Supplement to the Journal of Nematology
22:775-778.
Wescott, S. W., III, and Burrows, P. M. (1991). Degree-day models for predicting egg hatch and population increase of Criconemella xenoplax. Journal of Nematology 23, 386-392.
Return to Plant Parasites Menu